SCEMBLIX can be an option for adults with previously treated Ph+ CML in chronic phase
If you've taken another tyrosine kinase inhibitor (TKI) for Ph+ CML in chronic phase but your treatment isn’t working as you’d hoped, it may be time to ask about SCEMBLIX.
Here is more information about how SCEMBLIX compares to Bosulif* in patients with Ph+ CML in chronic phase previously treated with 2 or more TKIs.
In a head-to-head clinical study of adult patients with Ph+ CML in chronic phase previously treated with 2 or more TKIs:
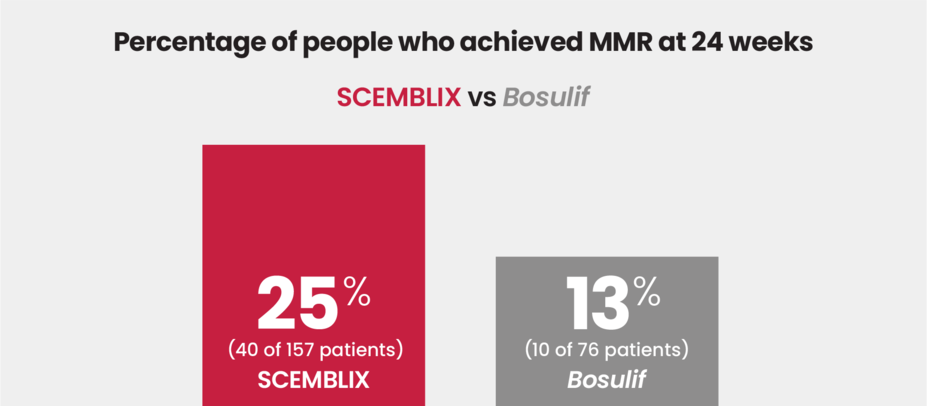
Nearly 2x more patients achieved MMR with SCEMBLIX vs Bosulif at 24 weeks
Major molecular response (MMR) is a treatment goal that is reached when the level of BCR::ABL1 gene in the blood is ≤0.1% of the level measured at your CML diagnosis.
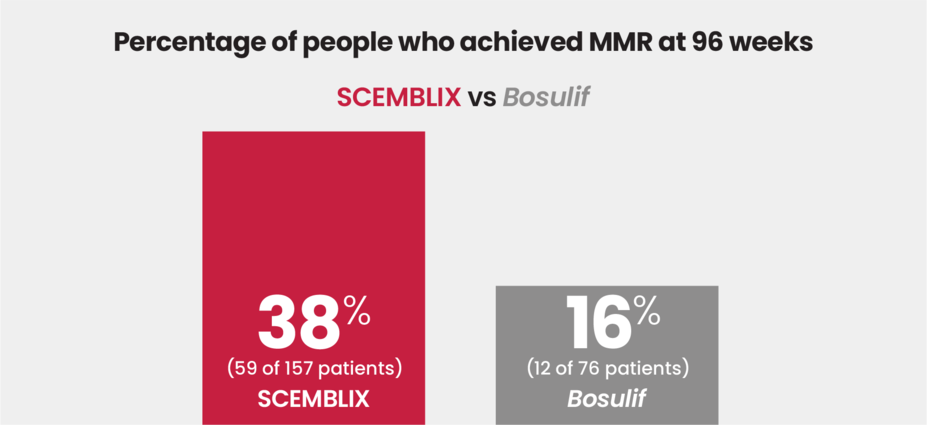
SCEMBLIX is the only treatment for Ph+ CML in chronic phase to show superior efficacy, such as response rates, vs Bosulif in a clinical trial.
At 96 weeks, more than 2x as many patients achieved MMR with SCEMBLIX vs Bosulif
In the same clinical study among patients with Ph+ CML in chronic phase who were previously treated with 2 or more TKIs:
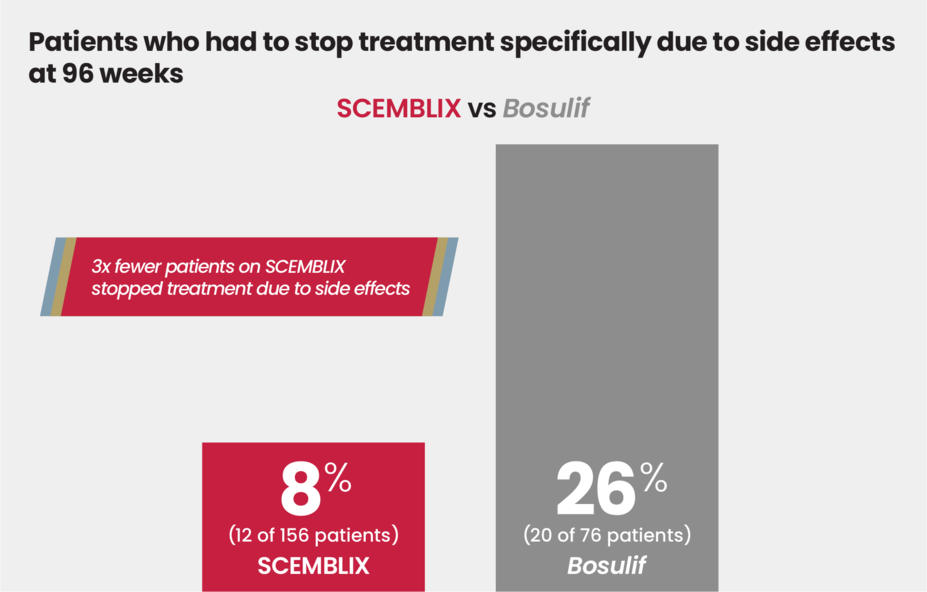
3x fewer patients on SCEMBLIX stopped treatment due to side effects at 96 weeks
In the same clinical study among patients with Ph+ CML in chronic phase who were previously treated with 2 or more TKIs:
Common side effects
In this clinical trial, the most common side effects reported (≥ 20%) with SCEMBLIX were nose/throat infections; pain in the muscles, bones, or joints; headache; and tiredness.
These are not all of the possible side effects of SCEMBLIX.
Review the serious and most common side effects of SCEMBLIX. Learn more
Take an active role in your treatment and ask about SCEMBLIX |
Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase; TKIs, tyrosine kinase inhibitors.
*Bosulif is a registered trademark of Pfizer Inc.
†Limitations apply. Valid only for those with private insurance. The Program includes the Co-Pay Plus offer, Plus Card (if applicable), and Rebate, with a combined annual limit. Patient is responsible for any costs once limit is reached in a calendar year. Program not valid (i) under Medicare, Medicaid, TRICARE, VA, DoD, or any other federal or state healthcare program, (ii) where patient is not using insurance coverage at all, (iii) where the patient’s insurance plan reimburses for the entire cost of the drug, or (iv) where product is not covered by patient’s insurance. The value of this program is exclusively for the benefit of patients and is intended to be credited towards patient out-of-pocket obligations and maximums, including applicable co-payments, coinsurance, and deductibles. Program is not valid where prohibited by law. Patient may not seek reimbursement for the value received from this program from other parties, including any health insurance program or plan, flexible spending account, or healthcare savings account. Patient is responsible for complying with any applicable limitations and requirements of their health plan related to the use of the Program. Valid only in the United States and Puerto Rico. This Program is not health insurance. Program may not be combined with any third-party rebate, coupon, or offer. Proof of purchase may be required. Novartis reserves the right to rescind, revoke, or amend the Program and discontinue support at any time without notice.